Given the following standard reduction potentials, 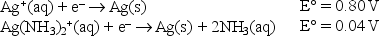 calculate the formation constant of Ag(NH3) 2+ at 25°C.
calculate the formation constant of Ag(NH3) 2+ at 25°C.
Definitions:
Registered Nurse
A healthcare professional licensed to practice nursing, offering critical health care and support.
Professional Nurse
A licensed healthcare professional skilled in providing patient care, education, and advice based on extensive training in nursing.
Essential Oils
Concentrated extracts from plants, used for aroma or therapeutic purposes.
Aromatherapy
The practice of using essential oils and other aromatic plant compounds to improve psychological or physical wellbeing.
Q8: Esters are synthesized from two classes of
Q19: What concentration of Ni<sup>2+</sup> ion remains in
Q35: The following amino acids all have nonpolar
Q46: Complete and balance the nuclear equation <img
Q48: How old is a bottle of wine
Q65: For a certain reaction, <span
Q66: What mass of sodium nitrite must be
Q72: Tritium is a radioisotope of hydrogen
Q76: Will a precipitate (ppt)form when 300.mL of
Q100: Arrange the acids HBr, H<sub>2</sub>Se, and H<sub>3</sub>As