For the reaction 3Fe(s) + C(s) 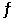 Fe3C(s) , H° = 21 kJ/mol and S° = 20.4 J/mol·K at 25°C. Estimate the minimum temperature above which the formation of cementite (Fe3C) is favored.
Fe3C(s) , H° = 21 kJ/mol and S° = 20.4 J/mol·K at 25°C. Estimate the minimum temperature above which the formation of cementite (Fe3C) is favored.
Definitions:
Acquisition
The process of learning or obtaining new information, skills, or responses.
Good Mood
A positive emotional state characterized by feelings of happiness, contentment, and well-being.
Stimulus Discrimination
The learned ability to differentiate between similar stimuli and respond accordingly in conditioning.
Observational Learning
A learning process whereby individuals acquire new behaviors or information by watching the actions of others, also known as social learning or modeling.
Q17: The total number of electrons in the
Q18: The polymer formed from the monomer CH<sub>2</sub>=CH-CN
Q21: Smith Inc wishes to use the revaluation
Q27: A possible reaction leading to removal
Q38: Of the three oxides SiO<sub>2</sub>, MgO, and
Q52: In the <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="In the
Q74: In the following reaction, identify X. <img
Q86: Explain how an impairment loss is allocated
Q95: The pH of a solution that is
Q105: How many moles of H<sub>2</sub> are