Which would you expect to have a higher melting point: sodium chloride, NaCl, or aluminum oxide, 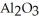 ?
?
Definitions:
Depression
A mental health disorder characterized by persistent feelings of sadness, loss of interest, and a lack of motivation.
Advantages and Disadvantages
The positive and negative aspects or outcomes associated with a particular choice, decision, or action.
Clinical Psychologists
are professionals specializing in diagnosing and treating mental health disorders, utilizing a variety of therapy methods and psychological tests.
Nonclinical Areas
Fields or specialties within health and psychology that do not involve direct patient care or treatment.
Q73: A minimum temperature exists (absolute zero). Why
Q87: Why might softened water not be good
Q91: Which of the following would not be
Q91: What is transmutation?<br>A)changing one element into another
Q91: Which would have the most kinetic energy?
Q99: How would you classify the following material?
Q109: Which of the following would have the
Q121: A cohesive force is best described as
Q140: Given the following generic chemical reaction, which
Q146: If an atom were the size of