Consider the electron-dot geometries of P  and S
and S 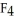 . Which do you expect is the more polar compound?
. Which do you expect is the more polar compound?
Definitions:
Bribery
The act of offering, giving, receiving, or soliciting something of value to influence the action of an official or other person in charge of a public or legal duty.
Worker Safety
The practices, policies, and regulations designed to protect employees from risks and hazards associated with their work environment.
Supply Availability
The extent to which goods, services, and materials needed by consumers or businesses are available for purchase or delivery.
Supply Research
The process of studying the market and suppliers to understand the availability, quality, and cost of materials or services needed by a business.
Q2: Account for the observation that ethyl alcohol,
Q21: Which has more atoms: 64.058 g of
Q55: If nitrogen, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2711/.jpg" alt="If nitrogen,
Q62: Which equation best describes the reaction represented
Q78: Neon, Ne, (number 10)has a relatively large
Q86: What is happening at the molecular level
Q111: Your inventor friend proposes a design of
Q139: If it takes three golf balls to
Q139: Why does soap decrease the surface tension
Q139: How are oxygen molecules attracted to water