Water, 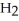 O, and methane, C
O, and methane, C 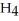 , have about the same mass and differ by only one type of atom. Why is the boiling point of water so much higher than that of methane?
, have about the same mass and differ by only one type of atom. Why is the boiling point of water so much higher than that of methane?
Definitions:
Eye Color
The color of the iris, which can range widely among individuals, including blue, brown, green, and varying shades thereof.
Short-Term Memory
The capacity for holding a small amount of information in an active, readily available state for a short period of time.
Unlimited Capacity
A theoretical concept in which a system or process is considered to have no bounds or limits in its ability to hold or process information.
Long-Term Memory
A type of memory capable of storing large amounts of information for potentially unlimited durations, including facts, experiences, and skills.
Q12: How does a solute such as salt
Q17: A saturated solution of compound X in
Q19: Which of the following statements is true?<br>A)The
Q32: The relative mass of carbon is 3/8
Q34: For the following reaction, identify whether the
Q39: Half-frozen fruit punch is always sweeter than
Q68: Describe what happens to an ice cube
Q82: A walnut stuck to a pin is
Q95: Which of the following solutions is the
Q137: Which of the following determined the ratio