Which would you expect to have a higher melting point: sodium chloride, NaCl, or aluminum oxide, 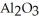 ?
?
Definitions:
Id
In psychoanalytic theory, the part of the mind that is the source of instinctive impulses and demands for immediate satisfaction of primitive needs.
Ego
In Freudian theory, the part of the personality that mediates among the demands of the id, the superego, and reality.
Superego
In psychoanalytic theory, the part of a person's mind that acts as a self-critical conscience, reflecting social standards learned from parents and teachers.
Projective Tests
Psychological assessment tools that use ambiguous stimuli to elicit responses that reveal aspects of an individual's personality.
Q41: Which of the following statements regarding a
Q54: Which of the following is not a
Q95: Water is most dense at _.<br>A)-4°C<br>B)0°C<br>C)+4°C<br>D)+100°C
Q106: An electron in the outermost shell of
Q107: Which of the following physical properties would
Q125: What is wrong with the following depiction
Q127: What happens if you were to place
Q127: Why can you determine wind direction by
Q139: Why are metal ores so valuable?<br>A)They are
Q145: Which of the following has the greatest