Consider the electron-dot geometries of P  and S
and S 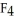 . Which do you expect is the more polar compound?
. Which do you expect is the more polar compound?
Definitions:
Long-Term Orientation
A cultural or organizational perspective that emphasizes planning and investing in the future, valuing perseverance, and viewing change as evolutionary.
Ethical Decision Making
The process of evaluating and choosing among alternatives in a manner consistent with ethical principles.
Government Laws
Consist of legal rules and regulations established by governmental entities, which individuals and organizations are obliged to follow.
Greatest Good
A principle often associated with utilitarianism, focusing on actions that result in the maximum benefit or happiness for the largest number of people.
Q24: How many chloride ions (Cl<sup>1-</sup>)are needed to
Q28: A sealed plastic bottle is filled with
Q31: Which would you expect to have a
Q42: To impart a hickory flavor to a
Q85: Why are the atomic masses listed in
Q89: If you have one molecule of CO<sub>2,</sub>
Q103: What is molarity?<br>A)the number of moles of
Q105: A thin stream of water is pulled
Q116: Heat is absorbed by a substance when
Q137: The neon atom tends NOT to gain