Water, 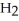 O, and methane, C
O, and methane, C 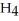 , have about the same mass and differ by only one type of atom. Why is the boiling point of water so much higher than that of methane?
, have about the same mass and differ by only one type of atom. Why is the boiling point of water so much higher than that of methane?
Definitions:
Performance Measurement
A method used by organizations to evaluate and assess the efficiency and effectiveness of its operations, often by analyzing quantitative data.
Promotion
The advancement of an employee to a higher position or job title within a company.
Instrumentality
The belief or expectation that performing a given behavior is associated with a particular outcome.
Performance Impact
The effect or influence that an individual's or group's actions have on the overall outcomes or goals of a project or organization.
Q4: Which of the following statements about radiation
Q8: When <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2711/.jpg" alt="When Po
Q26: How are frequency and wavelength related?<br>A)The higher
Q41: If the relative mass of a pingpong
Q52: A soap molecule is _.<br>A)primarily polar<br>B)primarily nonpolar<br>C)both
Q63: An element found in another galaxy exists
Q79: When blue food coloring is dissolved in
Q99: How would you classify the following material?
Q102: Is the mass of an atomic nucleus
Q135: Two candles of the same mass, one