Based on atomic size, which would you expect to be more soluble in water: helium, He, or nitrogen, 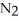 ?
?
Definitions:
Customer Tastes
Preferences or inclinations of consumers, which influence their buying behaviors and choice of products or services.
Historical Data
Previously recorded information that is used to understand past events or trends, often used in analysis or research to predict future outcomes.
Pilot Experiment
A small-scale study conducted to assess the feasibility of a product or service.
Feasibility
The measure of how possible or practical the execution of a project, plan, or idea is.
Q7: Why do water drops bead on a
Q26: How are frequency and wavelength related?<br>A)The higher
Q88: Is it at all possible for a
Q95: Which has the greater mass, 1.204 ×
Q105: What property of half-lives makes radioactive material
Q111: How many grams of water, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2711/.jpg"
Q115: If a neutral element has 8 neutrons
Q116: Uranium-235 releases an average of 2.5 neutrons
Q145: What is a hydrogen bond?<br>A)a special type
Q148: The _ is what needs to be