Why does water not freeze at 0°C when either ions or molecules other than 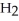 O are present?
O are present?
Definitions:
Strategic Planning
Strategic planning involves determining the course an organization will take and deciding how to allocate resources, such as money and personnel, to follow that path.
Forecasting
the process of making predictions about future events based on historical and current data.
Retirement Account
A financial arrangement designed to replace employment income upon retirement, often offering tax benefits.
Trend Analysis
The practice of collecting information and attempting to spot a pattern, often used in financial markets, research, and responsive business strategy planning.
Q18: Which of the following statements best describes
Q21: Which of the following molecules contains a
Q41: The boiling point of 1,4-butanediol is 230°C.
Q44: Would fevers run higher or lower if
Q58: Alpha and beta rays are deflected in
Q77: Which of the following best describes a
Q89: The valence electron of a sodium atom
Q132: What is the mass of one mole
Q141: Passing arsenic contaminated water through a long
Q149: Phosphate ions, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2711/.jpg" alt="Phosphate ions,