Why is calcium chloride, 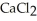 , more effective at melting ice than sodium chloride, NaCl?
, more effective at melting ice than sodium chloride, NaCl?
Definitions:
Price Taker
A buyer or seller that is unable to influence the market price of a product or service.
Marginal Revenue Curve
A graphical representation showing how the revenue from selling one more unit of a good or service changes as production volume changes.
Price-searcher Firm
A company that has the ability to set the price for its products because it does not face perfect competition.
Marginal Revenue
The increase in revenue that results from the sale of one additional unit of product.
Q3: Which of the following would cause the
Q23: If the oceans became acidic, they would
Q24: How many chloride ions (Cl<sup>1-</sup>)are needed to
Q26: What might the relationship be between an
Q35: How is the junction barrier between n-type
Q49: How many valence electrons does bromine (Br,
Q60: Why is it so easy for a
Q110: How does a catalyst increase the rate
Q118: Cells at the top of a tree
Q134: It easier to cool off on a