Why does water not freeze at 0°C when either ions or molecules other than 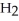 O are present?
O are present?
Definitions:
Fiscal Policy
The use of government spending and taxation to influence the economy, including policies aimed at reducing unemployment, controlling inflation, and fostering economic growth.
Election Year
A year in which a formal decision-making process is conducted through which citizens vote to choose public officials or to decide on public policy issues.
Government Purchases
Government purchases comprise spending by government bodies on goods and services that contribute directly to the economy's aggregate demand.
Stagflation
A period of slow economic growth and high unemployment while prices continue to rise.
Q4: Which of the following statements about desalinization
Q14: Why might the following nuclear reaction not
Q19: Which type of radiation is being emitted
Q41: Which of the following statements regarding a
Q44: Which is higher in an endothermic reaction:
Q51: An individual carbon-oxygen bond is polar. Yet
Q53: Which of the following might have the
Q100: Qualitatively, what happens to the hydroxide ion
Q120: The following set of redox reactions takes
Q140: How is the number of unpaired valence