Why is calcium chloride, 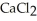 , more effective at melting ice than sodium chloride, NaCl?
, more effective at melting ice than sodium chloride, NaCl?
Definitions:
Spotify
A digital music service that provides access to millions of songs, podcasts, and videos from artists all over the world.
Apple Music
A music and video streaming service developed by Apple Inc., allowing users to listen to their music library and access a vast library of songs and playlists.
Great Firewall
A term used to describe the strict internet censorship and surveillance employed by the Chinese government to regulate the internet within China.
Block List
A list of domains, IP addresses, or other identifiers that are denied access to a certain resource or service, often used to prevent spam or unauthorized access.
Q2: Which of the following would have the
Q2: Why do we use the pH scale
Q8: Which of the following molecules has a
Q14: Which of the following compounds would be
Q34: The half-life of carbon-14 is 5,730 years.
Q50: Which of the following statements describes a
Q70: It is relatively easy to pull an
Q132: How is the solubility of a solid
Q140: Given the following generic chemical reaction, which
Q151: How many molecules of sucrose are in