Why does iodine, 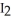 (s) , spontaneously sublime at room temperature?
(s) , spontaneously sublime at room temperature?
Definitions:
Carroll Izard
An influential American psychologist known for his contributions to the study of emotions and the development of the Differential Emotions Theory.
Basic Emotions
Fundamental human emotions that are universally experienced, such as happiness, sadness, fear, and anger.
Nonverbal Threats
Communications or signals that do not use words but are intended to convey a warning or threat.
Lies Detection
The process of identifying whether a person is not telling the truth through various means.
Q2: Account for the observation that ethyl alcohol,
Q26: If it takes energy to break bonds
Q37: Which of the following molecules should have
Q53: Which of the following molecules is not
Q92: A student is told to use 10.00
Q113: Given the following specific heats and that
Q116: Which of the above structures should be
Q122: How many grams of sodium chloride are
Q126: Which of the following molecules could be
Q133: Why are ion-dipole attractions stronger than dipole-dipole