How many moles of water, 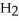 O, are produced from the reaction of 16 grams methane, C
O, are produced from the reaction of 16 grams methane, C 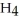 , with an unlimited supply of oxygen,
, with an unlimited supply of oxygen, 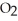 . How many grams of
. How many grams of 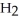 O is this? C
O is this? C 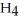 + 2
+ 2 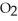 → C
→ C 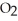 + 2
+ 2 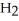 O
O
Definitions:
Exporting Goods
The process of selling and shipping goods from one's own country to foreign markets.
Seeking New Markets
The strategy of exploring and entering new geographic or demographic areas to expand business operations and customer base.
Workforce Planning
A strategic approach to forecasting the current and future workforce needs of an organization to achieve its goals.
Human Capital
The total capabilities, knowledge, and non-material assets belonging to individuals that have the capability to produce monetary value for the people themselves, their employers, or their surrounding community.
Q8: The n-type/p-type junction is like _.<br>A)a revolving
Q26: Why does oxygen have such a low
Q27: Which has the greatest number of atoms?<br>A)28
Q28: Which element is closer to the upper
Q57: Describe how a boiling liquid can be
Q79: Zinc, Zn, is more easily oxidized than
Q79: Hard water contains excessive amounts of _.<br>A)fluoride
Q98: Does a plastic bottle of fresh water
Q103: What is molarity?<br>A)the number of moles of
Q119: What is the purpose of treating water