Use the bond energies below to determine whether the following reaction is exothermic or endothermic: 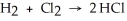 H-H (bond energy: 436 kJ/mol) Cl-Cl (bond energy: 243 kJ/mol)
H-H (bond energy: 436 kJ/mol) Cl-Cl (bond energy: 243 kJ/mol)
H-Cl (bond energy: 431 kJ/mol)
Definitions:
Openness
One of the five major dimensions of personality, characterized by an appreciation for art, emotion, adventure, and unusual ideas; imaginative and curious individuals.
Agreeableness
A personality trait characterized by compassion, cooperativeness, and a desire to maintain positive social relations.
Conscientiousness
A personality trait characterized by diligence, carefulness, and a strong sense of duty.
Unconscious Processes
Mental activities below the level of conscious awareness that influence thoughts, feelings, and behaviors.
Q7: Which of the following is not a
Q25: What is an endothermic reaction?<br>A)It is a
Q32: Why is calcium fluoride, Ca <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2711/.jpg"
Q37: What is the number of moles of
Q41: How does p-type silicon differ from pure
Q45: Barium ions carry a 2+ charge, and
Q45: Why does sweat cool you off?<br>A)The water
Q65: Which of the above structures should be
Q104: During osmosis _.<br>A)the water moves into the
Q147: Which has more atoms: 17.031 g of