Why does iodine, 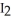 (s) , spontaneously sublime at room temperature?
(s) , spontaneously sublime at room temperature?
Definitions:
Effort
The use of physical or mental energy to achieve a goal or complete a task.
Three-Person Trip
an outing or journey undertaken by a group of three individuals together.
Gordon Allport
An influential American psychologist known for his extensive research in personality and the psychology of prejudice.
Attitudes
Global evaluations toward some object or issue
Q15: How does the purchase and use of
Q21: Which of the following molecules contains a
Q71: What is the main difference between a
Q85: Which of the following statements best describes
Q90: Distinguish between a metal and a metal-containing
Q91: Why is it better to recycle metals
Q105: Shown below is the line structure of
Q109: Sodium bicarbonate, NaHC <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2711/.jpg" alt="Sodium bicarbonate,
Q127: Which of the following two stick structures
Q133: A gold ring cannot sit on the