How readily an acid donates a hydrogen ion is a function of how well the acid is able to accommodate the resulting negative charge it gains after donating. Which should be the stronger acid: water or hypochlorous acid? Why? 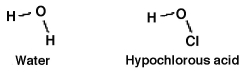
Definitions:
Free-rider Problem
A situation where individuals benefit from resources, goods, or services that they do not pay for, which can lead to underproduction or depletion of those resources.
Rivalry
The competitive relationship between firms in the market, striving to gain advantage over each other in terms of sales, market share, or innovation.
Nonexcludable
A characteristic of a good or service whereby it is not possible to prevent people who have not paid for it from having access to it.
Free-rider Problem
A situation in which individuals benefit from resources, goods, or services without paying for them, leading to underprovision of those goods or services.
Q6: For the following acid-base reaction, identify what
Q30: Why is it easier for the body
Q30: Correctly identify the following functional groups in
Q36: For the following acid-base reaction, identify what
Q92: The following set of redox reactions takes
Q106: Why do water heaters lose their efficiency
Q125: The following statement describes what level of
Q126: Given that the above energy profiles have
Q127: What happens if you were to place
Q149: Is it possible to have a macroscopic