Some molecules are able to stabilize a negative charge by passing it from one atom to the next by a flip-flopping of double bonds. This occurs when the negative charge is one atom away from an oxygen double bond as follows. Note that the curved arrows indicate the movement of electrons: 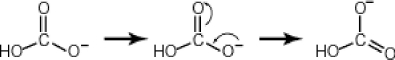 Why then is sulfuric acid so much stronger an acid than carbonic acid?
Why then is sulfuric acid so much stronger an acid than carbonic acid? 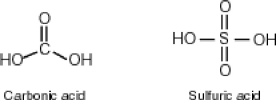
Definitions:
Null Hypothesis
In statistical analysis, the hypothesis that there is no effect or no difference, and any observed difference is due to sampling or experimental error.
Independent Variable
In an experiment, the variable that is manipulated or changed by the researcher to observe its effect on the dependent variable.
Research Hypothesis
A specific, testable prediction about the relationship between at least two variables in a population, based on theory or previous research.
Independent Variable
A variable that is manipulated by the researcher to observe its effect on the dependent variable.
Q3: What would be the concentration of hydronium
Q6: What property primarily determines the effect of
Q34: A battery operates by _.<br>A)oxidation<br>B)reduction<br>C)both oxidation and
Q43: What is the number of grams of
Q49: The overall rate at which humans are
Q84: How many molecules of aspirin (formula mass
Q107: Shown below is the line structure of
Q108: How would connecting iron with a wire
Q109: Given the following energy profiles, which of
Q135: Which of the following is NOT a