Why is phosphoric acid, 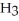
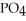 , a stronger acid than disodium hydrogen phosphate,
, a stronger acid than disodium hydrogen phosphate, 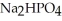 ?
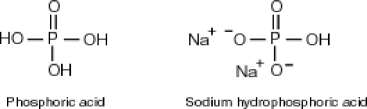
? 
Definitions:
Guidelines
Recommendations or protocols aimed at steering actions or decisions in a specific direction or towards achieving a desired outcome.
Family Member
An individual who is related to another by birth, adoption, or marriage, and holds a specific familial role such as parent, sibling, or spouse.
Nonfamily Employees
Employees who are not relatives or members of the business owner's family, hired to perform tasks and roles within an organization.
Financial Condition
The overall health of an organization's finances, including its assets, liabilities, and equity.
Q12: Which of the following is most likely
Q29: Iron atoms have a greater tendency to
Q30: Which of the following is not a
Q34: For the following reaction, identify whether the
Q79: Hard water contains excessive amounts of _.<br>A)fluoride
Q86: What is happening at the molecular level
Q94: Which of the above reactions would proceed
Q100: Qualitatively, what happens to the hydroxide ion
Q120: The separation of charges within a polar
Q144: What property of nitrocellulose was exploited in