Multiple Choice
Why are aqueous solutions of highly charged metal ions, such as 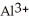 , usually acidic?
, usually acidic?
Definitions:
Bed and Breakfast
A lodging establishment that offers overnight accommodation and breakfast, typically in a private home or small hotel.
Related Questions
Q1: If all of the liquids pictured above
Q21: The formula for a certain ether is
Q28: The atomic masses appearing in the periodic
Q80: Aluminum metal undergoes the same basic corrosion
Q80: Which of the following molecules is a
Q95: Which of the following solutions is the
Q97: When the hydronium ion concentration equals 1
Q120: Why are Ping-Pong balls so highly flammable?<br>A)They
Q122: If the pH of a solution was
Q144: What property of nitrocellulose was exploited in