When a hydronium ion concentration equals 1 × 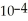 moles per liter, what is the pH of the solution? Is the solution acidic or basic?
moles per liter, what is the pH of the solution? Is the solution acidic or basic?
Definitions:
Pertinent Information
Information that is relevant and important to a specific context or subject.
In-Person Combination
A method that involves direct, face-to-face interaction integrated with other forms of communication or activities.
Immediate Feedback
Instant reactions or responses provided during or right after a task or activity, valuable for learning and improvement.
Verifiable Record
Documented evidence or data that can be checked or validated for accuracy and authenticity.
Q22: Red blood cells have a high concentration
Q25: Besides genetic information, what is the main
Q30: Why is it easier for the body
Q56: The following set of redox reactions takes
Q79: According to the lock-and-key model, in order
Q109: Given the following energy profiles, which of
Q120: Steel wool wetted with vinegar is sealed
Q132: The general chemical equation for photosynthesis is
Q133: What type of polymer would be best
Q137: Which of the following molecules contains a