When the pH of a solution is 1, the concentration of hydronium ions is 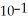 M, which is the same as 0.1 M. Assume that the volume of this solution is 500 mL and that the solution is not buffered. What would be the new pH of this solution after 500 mL of pure water is added? You will need a calculator with a log function to answer this question.
M, which is the same as 0.1 M. Assume that the volume of this solution is 500 mL and that the solution is not buffered. What would be the new pH of this solution after 500 mL of pure water is added? You will need a calculator with a log function to answer this question.
Definitions:
Communicating
The process by which individuals convey information, ideas, or feelings to one another through speech, writing, or other forms of expression.
Claim Response
The reaction or reply to a statement or assertion, particularly in the context of addressing a complaint or issue.
Personal Responsibility
The obligation to take ownership of one's actions and their consequences, acknowledging the personal role in outcomes.
Routine Messages
Regular or standard communications that occur within typical business or personal contexts, often following a set format or content.
Q4: What is the main structural feature that
Q29: Which of the following has the greatest
Q55: Capillary action causes water to climb up
Q58: Why does ice form at the surface
Q64: When the hydronium ion concentration equals 10
Q89: Which of the following is the hallmark
Q94: Why might a change in pH cause
Q95: As the hydronium ion concentration increases, the
Q97: Which of the following compounds is likely
Q126: Fatty acid molecules can also align to