How readily an acid donates a hydrogen ion is a function of how well the acid is able to accommodate the resulting negative charge it gains after donating. Which should be the stronger acid: water or hypochlorous acid? Why? 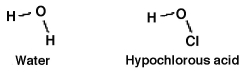
Definitions:
False Imprisonment
The unlawful confinement of a person without their consent or legal justification.
Ignorance of the Law
A principle stating that not knowing a law does not exempt individuals from being liable for violating it.
False Imprisonment
is the unlawful restraint of an individual's freedom of movement without legal justification.
Trespass Against the Person
Refers to a legal term involving direct or immediate harm or interference with a person's body or rights, such as assault or battery.
Q31: What does the value of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2711/.jpg"
Q49: If the relative mass of a hydrogen
Q59: Vitamins such as vitamin B and vitamin
Q73: Given the following diagram, describe what happens
Q85: If you keep a mixture of water
Q87: Which of the following is a heteroatom?<br>A)O<br>B)C<br>C)H<br>D)A
Q93: Hydrocarbons release a lot of energy when
Q98: Which is greater: 1.01 amu of hydrogen
Q143: How many structural isomers are shown here?
Q145: What is a hydrogen bond?<br>A)a special type