The amphoteric reaction between two water molecules is endothermic, which means that this reaction requires the input of energy in order to proceed: Energy + 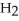 O +
O + 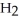 O →
O → 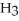
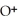 + O
+ O 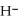 The warmer the temperature of the water, the more thermal energy is available for this reaction, and the more hydronium and hydroxide ions are formed. Based on this information, should the value of
The warmer the temperature of the water, the more thermal energy is available for this reaction, and the more hydronium and hydroxide ions are formed. Based on this information, should the value of 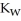 be expected to increase, decrease, or stay the same with increasing temperature?
be expected to increase, decrease, or stay the same with increasing temperature?
Definitions:
Forges
The act of creating counterfeit copies of documents, art, or other items with the intent to deceive; also refers to workshops equipped with a forge for metalworking.
Effective
Producing the intended or desired result.
Dishonors
Refers to the failure to fulfill an obligation, especially the failure of a bank to pay a check or note when presented.
Sufficient Funds
The necessary amount of money available to complete a transaction or meet financial obligations.
Q3: If the relative mass of a pingpong
Q34: Which of the following is not a
Q34: A battery operates by _.<br>A)oxidation<br>B)reduction<br>C)both oxidation and
Q35: Alkaloid salts are not very soluble in
Q47: What is an acid?<br>A)anything that donates hydrogen
Q48: What happens to the pH of soda
Q63: At what point will a buffer solution
Q90: Which of the half-reactions below would be
Q120: Why are Ping-Pong balls so highly flammable?<br>A)They
Q152: Which of the following statements describes structural