When a hydronium ion concentration equals 1 × 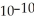 moles per liter, what is the pH of the solution? Is the solution acidic or basic?
moles per liter, what is the pH of the solution? Is the solution acidic or basic?
Definitions:
Future Expectations
The anticipations or outlook that consumers and businesses have about economic conditions that affect their spending and investment decisions.
Deferring Consumption
The act of postponing spending money on consumption goods to save or invest for future use.
Interest
The cost of borrowing money, usually expressed as a percentage of the amount borrowed, paid over a specific period.
Dividend
A portion of a company's earnings distributed to shareholders, usually in the form of cash or additional shares.
Q8: According to the following reaction, which molecule
Q46: If you saw the label "phenylephrine HCl"
Q48: What is the leading cause of drug
Q50: If an alcoholic beverage is 20 percent
Q88: Balance the following chemical equation. _ N<sub>2</sub>
Q88: If the specific heat of gold is
Q90: From the diagram above describing the phase
Q97: In one type of fuel cell, the
Q108: Metabolism includes _.<br>A)catabolism<br>B)anabolism<br>C)cellular respiration<br>D)all of the above
Q130: Which of the above stick structures would