When the pH of a solution is 1, the concentration of hydronium ions is 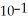 M, which is the same as 0.1 M. Assume that the volume of this solution is 500 mL and that the solution is not buffered. What would be the new pH of this solution after 500 mL of pure water is added? You will need a calculator with a log function to answer this question.
M, which is the same as 0.1 M. Assume that the volume of this solution is 500 mL and that the solution is not buffered. What would be the new pH of this solution after 500 mL of pure water is added? You will need a calculator with a log function to answer this question.
Definitions:
Nondirective
A type of therapy or counseling in which the therapist provides minimal guidance, allowing the client to direct the conversation.
Physical Appearance
The outward look or aspects of an individual or object, often the first noticeable thing in social interactions.
Eclectic
Incorporating ideas, styles, or concepts from a broad and diverse range of sources.
Psychological Theories
Systematic frameworks for understanding, predicting, and often altering human behavior and mental processes.
Q13: Boiling is when the _.<br>A)vapor pressure of
Q24: Which of the following might have the
Q32: Proteins are _ that have some biological
Q58: The general outcome of catabolism is _.<br>A)the
Q65: Which of the following statements best describes
Q82: What happens to the corrosive properties of
Q94: Hydrogen sulfide, H<sub>2</sub>S, burns in the presence
Q101: Can a catalyst react with a reactant?
Q134: Which of the above illustrations shows an
Q135: Rust has a tendency to form when