Consider the following molecules. What is the relationship between the degree to which the molecule is oxidized and its polarity? 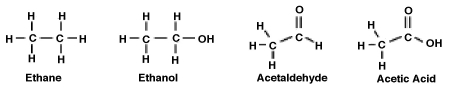
Definitions:
Sample
A subset selected from a larger population for the purpose of statistical analysis.
Null Hypothesis
A statement postulating that there is no difference or effect, used as a starting assumption in hypothesis testing.
Type I
A statistical error that occurs when a true null hypothesis is incorrectly rejected, also known as a "false positive."
Significance Level
Another term for level of significance, indicating the critical probability level at which the results of a statistical test are deemed significant.
Q8: At one time, halothane, C <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2711/.jpg"
Q11: How many electrons are gained or lost
Q16: How do most vasodilators work?<br>A)They release nitric
Q21: The formula for a certain ether is
Q27: How is cancer different from other diseases
Q47: What is an acid?<br>A)anything that donates hydrogen
Q61: Which of the following materials is most
Q67: Relative to pure water, an aqueous sugar
Q77: Which is better for you: a drug
Q83: Why does a glowing splint of wood