Why is the formation of iron hydroxide, Fe 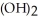 , from
, from 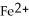 and O
and O 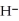 not considered an oxidation-reduction reaction?
not considered an oxidation-reduction reaction?
Definitions:
Economic Profit
The surplus remaining after deducting both explicit and implicit costs from total revenues, emphasizing a firm's financial performance beyond just its accounting profit.
Accounting Profit
Accounting profit is the monetary gain calculated by subtracting total explicit costs from total revenue.
Sunk Cost
A cost that has already been incurred and cannot be recovered, and therefore should not affect current and future business decisions.
Nonrefundable Ticket
A type of ticket for which the cost is not returned to the purchaser if they decide to cancel their booking or are unable to use the ticket.
Q13: Are the chemical reactions that take place
Q22: Which of the above molecules is a
Q23: Which of the following is a complete
Q66: What is the main characteristic of a
Q66: What is the formula mass of sulfur
Q73: How is a set of neurons different
Q74: Most fresh water lakes _.<br>A)contain no dissolved
Q128: How many oxygen molecules are needed to
Q135: Two candles of the same mass, one
Q144: What property of nitrocellulose was exploited in