Consider the following molecules. What is the relationship between the degree to which the molecule is oxidized and its polarity? 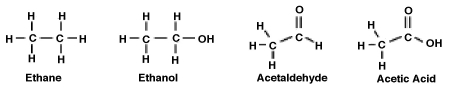
Definitions:
Light
A specific segment of the electromagnetic spectrum that can be seen by the human eye.
Wake-Sleep Cycles
The recurring patterns of sleep and wakefulness, reflecting the natural internal process that regulates the body's sleep need, typically aligned with the 24-hour day-night cycle.
Binocular Rivalry
A phenomenon in visual perception that occurs when each eye perceives a different image, leading to an alternation in awareness between the two images.
Brain Activity
The functioning and processes that occur within the brain, including neural activity, electrical impulses, and biochemical reactions.
Q4: Which bond would you rotate around to
Q5: Organic chemicals are so suitable for making
Q8: The n-type/p-type junction is like _.<br>A)a revolving
Q39: Why is a large quantity of water
Q41: If the relative mass of a pingpong
Q91: A buffer solution can neutralize _.<br>A)strong acids
Q110: How does ingested methanol lead to the
Q118: Cells at the top of a tree
Q122: The following set of redox reactions takes
Q139: If it takes three golf balls to