Multiple Choice
In the oxidation-reduction reaction Mg(s) + 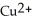 (aq) →
(aq) → 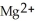 (aq) + Cu(s) , which atom or ion is reduced? Which atom or ion is oxidized?
(aq) + Cu(s) , which atom or ion is reduced? Which atom or ion is oxidized?
Definitions:
Related Questions
Q10: Glycogen is most structurally similar to _.<br>A)amylopectin<br>B)cellulose<br>C)amylose<br>D)pectin<br>E)chitin
Q26: Water is formed from the reaction of
Q38: Some molecules are able to stabilize a
Q61: Which of the following materials is most
Q80: What happens to two water molecules after
Q99: The sequence of amino acids within a
Q110: How does ingested methanol lead to the
Q119: A simple carbohydrate is _.<br>A)either a monosaccharide
Q119: Which of the above stick structures would
Q135: Two candles of the same mass, one