Why is 2,4,5-trifluorophenol much more acidic than phenol? 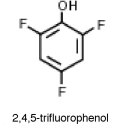
Definitions:
Normal Profits
The minimum level of profit needed for a company to remain competitive in the market, also seen as the opportunity cost of capital.
Purely Competitive Firm
A business operating in a market where there are many buyers and sellers, with none being able to influence prices significantly by their individual actions.
Costs
The value of resources consumed in the production of goods and services, including both fixed and variable expenses.
Differentiated Product
A product that is distinct in some way from others in the same category, allowing the seller to compete on factors other than price.
Q4: How many moles of H<sub>2</sub> bonds are
Q11: Which of the following statements accurately describes
Q35: According to the following reaction, which molecule
Q45: Sodium hydroxide is added to a buffer
Q52: Which of the following items would you
Q93: Hydrocarbons release a lot of energy when
Q97: In one type of fuel cell, the
Q121: In the following buffer system, what happens
Q123: Jewelry is often manufactured by electroplating an
Q133: Which of the above images would best