Explain why caprylic acid, C 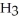 (C
(C 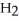
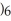 COOH, dissolves in a 5 percent aqueous solution of sodium hydroxide but caprylaldehyde, C
COOH, dissolves in a 5 percent aqueous solution of sodium hydroxide but caprylaldehyde, C  (C
(C 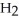
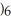 CHO, does not.
CHO, does not.
Definitions:
Nursing
A healthcare profession focused on the care of individuals, families, and communities to maintain or recover health and quality of life.
Diploma School
An educational institution that awards diplomas upon completion of specific courses of study.
Hospital
An institution where the sick or injured are given medical or surgical care.
British Colonies
Territories that were under the dominion and administration of the British Crown between the 16th and early 20th centuries.
Q20: Which of the following soil types would
Q31: How do anesthetics work to reduce pain?<br>A)prevent
Q43: Why is cancer treated most successfully in
Q70: How are the disinfecting properties of ultraviolet
Q70: The oxidation of iron to rust is
Q85: Which of the following would be considered
Q86: Which of the following elements would be
Q91: Which of the following molecules contains a
Q101: Which of the above images represents a
Q144: According to the following balanced chemical equation,