Given the data in the table below, ΔH°rxn for the reaction 2Ag2S (s) + O2 (g) → 2Ag2O (s) + 2S (s)
Is ________ kJ. 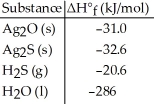
Definitions:
Price
The payment magnitude expected, called for, or compensated in exchange for something.
Law of Demand
A principle stating that all else being equal, as the price of a good or service increases, the quantity demanded decreases, and vice versa.
Inverse Relationship
A relationship between two variables in which one variable increases as the other decreases.
Quantity Demanded
The total amount of a good or service that consumers are willing to purchase at a specific price level.
Q19: In which set of elements would all
Q59: The average fuel value of sugars is
Q64: How many grams of H<sub>3</sub>PO<sub>4</sub> are in
Q96: Which one of the following statements is
Q105: Which of the following correctly lists the
Q107: What is the empirical formula of a
Q108: Which of the following correctly represents the
Q122: The wavelength of light emitted from a
Q175: The formula weight of silver chromate (Ag<sub>2</sub>CrO<sub>4</sub>)is
Q176: The lowest energy shell that contains f