Given the data in the table below and ΔH°rxn for the reaction SO2Cl2 (g) + 2H2O (l) → H2SO4 (l) + 2HCl (g) ΔH° = -62 kJ
ΔH°f of HCl (g) is ________ kJ/mol. 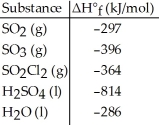
Definitions:
Conspicuous Consumption
The act of purchasing and using products and services to publicly display wealth and social status rather than to meet basic needs.
Sofia Coppola
An American film director, producer, and screenwriter known for her distinct narrative and visual styles, often exploring themes of loneliness, celebrity, and existential angst.
Costuming
The art and practice of designing, creating, and selecting costumes for characters in film, theater, and television to convey aspects of their personality or the setting.
Cyberpunk
A genre of science fiction set in a lawless subculture of an oppressive society dominated by computer technology.
Q39: Work equals force times distance.
Q61: For resonance forms of a molecule or
Q78: Which electron configuration represents a violation of
Q83: The element in the periodic table that
Q87: Of the following, which gives the correct
Q92: What volume (L)of 0.250 M HNO<sub>3 </sub>is
Q118: A tenfold dilution of a sample solution
Q154: When the following equation is balanced, the
Q164: What is the wavelength of light (nm)that
Q165: Of the metals below, only _ will