Given the data in the table below, ΔH°rxn for the reaction Ag2O (s) + H2S (g) → Ag2S (s) + H2O (l)
Is ________ kJ. 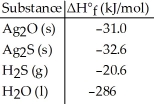
Definitions:
Physiology
The branch of biology that deals with the normal functions of living organisms and their parts.
Science
The systematic study of the structure and behavior of the physical and natural world through observation and experiment.
Social Level
refers to the degree of societal organization and integration among individuals within a community or society, impacting cultural, economic, and social dynamics.
Situational Factors
External factors in the environment that can influence an individual's behavior and decision-making processes.
Q3: The balanced reaction between aqueous potassium hydroxide
Q4: Which one of the following is a
Q11: What volume (mL)of 7.48 × 10<sup>-2</sup> M
Q14: Calculate the work (kJ)done during a reaction
Q23: Which of the following correctly represents the
Q46: A valid Lewis structure of _ cannot
Q74: How many grams of CH<sub>3</sub>OH must be
Q80: Of the bonds C-N, C <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2701/.jpg"
Q135: A 22.5-g sample of ammonium carbonate contains
Q141: The wavelength of a photon that has