Given the data in the table below, ΔH°rxn for the reaction 2SO2 (g) + O2 (g) → 2SO3 (g)
Is ________ kJ. 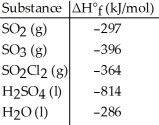
Definitions:
Depreciation
The planned allocation of a physical asset's cost over the time it remains useful.
Merchandising Business
A type of business that purchases finished goods and sells them to consumers, typically at a retail level.
Revenue Account
An account that records the income a company generates from its normal business operations.
Inventory Cost
The total cost incurred to acquire, hold, and convert raw materials into finished goods, including costs of purchase, handling, and storage.
Q26: _ is isoelectronic with argon, and _
Q40: The change in the internal energy of
Q47: Which one of the following is a
Q59: When the following equation is balanced, the
Q84: Which of the following is soluble in
Q104: How many grams of H<sub>3</sub>PO<sub>4</sub> are in
Q108: The units of specific heat are _.<br>A)K/J
Q134: The combustion of propane (C<sub>3</sub>H<sub>8</sub>)in the presence
Q139: The _ have the most negative electron
Q149: The electron configuration of a ground-state Ag