Using the table of bond dissociation energies, the ΔH for the following reaction is ________ kJ. 2HCl (g) + F2 (g) → 2HF (g) + Cl2 (g) 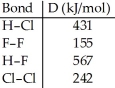
Definitions:
Irregular Chest Pain
describes chest discomfort that lacks a consistent pattern, potentially indicating a variety of health issues.
Stylus
A pen; the ECG writer.
Printed Representation
The physical manifestation of visual information on a surface, such as paper or fabric, usually through methods like printing or painting.
Dot Matrix Paper
Paper designed for use in dot matrix printers, characterized by its removable side margins with sprocket holes.
Q49: The O-S-O bond angle in SO<sub>2</sub> is
Q53: All of the halogens _.<br>A)exist under ambient
Q60: The n = 5 to n =
Q71: Arrange the following gases in order of
Q87: A sample of gas (1.9 mol)is in
Q88: Which one of the following is considered
Q130: The bond angle marked a in the
Q169: Which element would be expected to have
Q173: Oxides of the active metals combine with
Q175: What volume (L)of NH<sub>3</sub> gas at STP