Using the table of bond dissociation energies, the ΔH for the following reaction is ________ kJ. 2HCl (g) + F2 (g) → 2HF (g) + Cl2 (g) 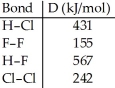
Definitions:
Multicellular Organism
Organism composed of interdependent cells that vary in their structure and function.
Pilus
A protein filament that projects from the surface of some prokaryotic cells.
Aerobic Respiration
A process in which organisms convert oxygen and glucose into energy, carbon dioxide, and water.
Mitochondrion
A membrane-bound organelle in cells that produces energy through oxidative phosphorylation, often referred to as the cell's powerhouse.
Q4: The noble gases were, until relatively recently,
Q15: The volume of hydrogen gas at 45.0
Q21: Renewable energy sources are essentially inexhaustible.
Q21: Based on the octet rule, boron will
Q48: The frequency of a photon that has
Q102: Which of the following is most likely
Q118: A mole of red photons of wavelength
Q123: The ion PO<sub>4</sub><sup>3- </sup>has _ valence electrons.<br>A)14<br>B)24<br>C)27<br>D)29<br>E)32
Q160: Gaseous mixtures _.<br>A)can only contain molecules<br>B)are all
Q180: The n = 8 to n =