Multiple Choice
The hybridization of the oxygen atom labeled x in the structure below is ________. 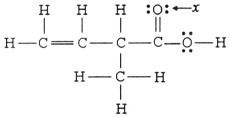
Definitions:
Related Questions
Q2: What is the predominant intermolecular force in
Q3: The oxide of which element below can
Q9: The _ quantum number defines the shape
Q16: Hydrogen is unique among the elements because
Q43: Which one of the following elements has
Q51: The properties of graphene include _.<br>A)high strength<br>B)low
Q95: Based on molecular orbital theory, there is/are
Q124: Why don't we draw double bonds between
Q132: What are the elements called that are
Q140: To convert from one resonance structure to