A gas vessel is attached to an open-end manometer containing a nonvolatile liquid of density 0.791 g/mL as shown below. 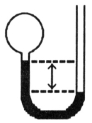 The difference in heights of the liquid in the two sides of the manometer is 43.4 cm when the atmospheric pressure is 755 mm Hg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is ________ atm.
The difference in heights of the liquid in the two sides of the manometer is 43.4 cm when the atmospheric pressure is 755 mm Hg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is ________ atm.
Definitions:
RAM
See random-access memory.
Local Vendor
A local individual or business that sells goods or services in a specific area or community.
Supply Companies
Businesses that provide materials, equipment, and supplies to consumers, businesses, or other organizations.
Asepsis
The state of being free from disease-causing microorganisms to prevent infection.
Q5: The heating curve shown was generated by
Q11: The strength of a covalent bond is
Q41: To produce maximum heat, an explosive compound
Q61: The monomer that is polymerized to make
Q70: On a clear day at sea level,
Q70: Using the table of average bond energies
Q92: Electron affinity measures how easily an atom
Q106: The Cl-Si-Cl bond angle in the SiCl<sub>2</sub>F<sub>2</sub>
Q110: The heating curve shown was generated by
Q141: The concentration of HCl in a solution