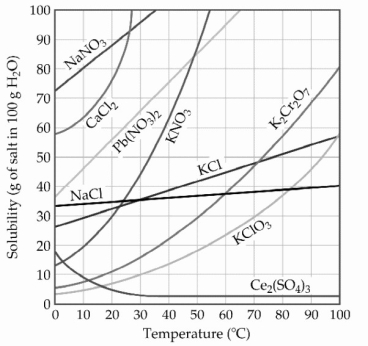
-The solubility of MnSO4 monohydrate in water at 20 °C is 70.0 g per 100.0 mL of water. A solution at 20 °C that is 4.22 M in MnSO4 monohydrate is best described as a(n) ________ solution. The formula weight of MnSO4 monohydrate is 168.97 g/mol.
Definitions:
Jazz
A music genre that originated in the African-American communities of New Orleans, characterized by strong rhythms, improvisation, and a blend of different musical traditions.
Analyze Reasons
The process of examining the causes or motives behind certain actions or events.
Alike
Similar or comparable in nature, characteristics, or appearance.
Ralph Waldo Emerson
A 19th-century American essayist, lecturer, philosopher, and poet who led the transcendentalist movement.
Q2: Which component of air is the primary
Q22: CO (5.00 g)and CO<sub>2</sub> (5.00 g)were placed
Q49: Which one of the following substances would
Q60: A sample of a gas originally at
Q68: A solution of ammonia is 2.0% ionized
Q68: If the reaction quotient Q for a
Q69: An ionic solid, NaCl (s), dissolves in
Q127: Compounds composed of a salt and water
Q128: A first-order reaction has a rate constant
Q137: The K<sub>a</sub> for HCN is 4.9 ×