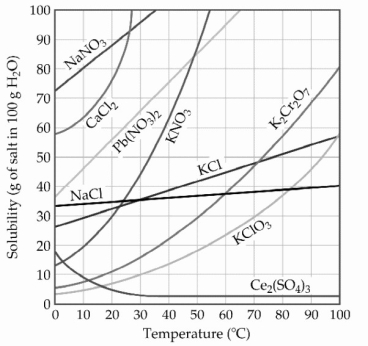
-The solubility of MnSO4 monohydrate in water at 20 °C is 70.0 g per 100.0 mL of water. A solution at 20 °C that is 0.401 M in MnSO4 monohydrate is best described as a(n) ________ solution. The formula weight of MnSO4 monohydrate is 168.97 g/mol.
Definitions:
Glomerulus
A network of tiny blood vessels located within the kidney that filters waste products from the blood to form urine.
Glomerular Capsule
A cup-shaped structure in the kidney that encases the glomerulus and initiates the filtration process of blood.
Efferent Arteriole
A small blood vessel that carries blood away from the glomerulus of the kidney after filtration has occurred.
Peritubular Capillaries
Tiny blood vessels that surround the tubules in the kidney, involved in reabsorption and secretion during urine formation.
Q10: Vulcanization involves heating rubber with sulfur dioxide
Q21: The K<sub>a</sub> for formic acid (HCO<sub>2</sub>H)is 1.8
Q22: The concentration of lead nitrate (Pb(NO<sub>3</sub>)<sub>2</sub>)in a
Q25: Which of the following statements is true?<br>A)Q
Q31: A solution is prepared by dissolving 15.0
Q34: A Br∅nsted-Lowry acid is defined as a
Q59: Consider the following reaction at equilibrium: 2CO<sub>2</sub>
Q107: The binding of molecules to the surface
Q130: Calculate the pOH of 0.606 M anilinium
Q138: A solution is prepared by dissolving 23.7