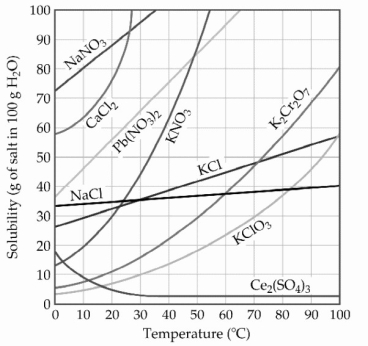
-The solubility of MnSO4 monohydrate in water at 20 °C is 70.0 g per 100.0 mL of water. A solution at 20 °C that is 4.22 M in MnSO4 monohydrate is best described as a(n) ________ solution. The formula weight of MnSO4 monohydrate is 168.97 g/mol.
Definitions:
Clay Tablets
Ancient writing materials made from clay, used primarily in Mesopotamia, for recording texts, transactions, or events.
Mesopotamia
An ancient region located in the eastern Mediterranean, broadly corresponding to present-day Iraq, northeastern Syria, southeastern Turkey, and parts of western Iran, known as the cradle of civilization.
Recordkeeping
The practice of maintaining and preserving records or documents detailing an organization's or individual's activities and transactions.
Bronze Metallurgy
The practice and knowledge involved in producing bronze, an alloy of copper and tin, used in tools, weapons, and sculptures in ancient times.
Q20: A solution contains 39% phosphoric acid by
Q32: Reaction rates are affected by reactant concentrations
Q42: A sample of oxygen gas (O<sub>2</sub>)was found
Q44: The decomposition of N<sub>2</sub>O<sub>5</sub> in solution in
Q50: A 0.333-g sample of an unknown pure
Q70: What are the principal organs that regulate
Q70: Potassium metal crystallizes in a body-centered cubic
Q77: Calculate the molality of a 24.4% (by
Q97: Which of the following expressions is the
Q154: A sample of potassium nitrate (49.0 g)is