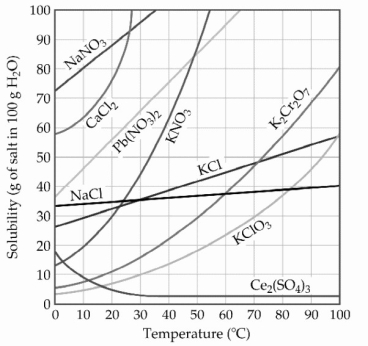
-The solubility of MnSO4 monohydrate in water at 20 °C is 70.0 g per 100.0 mL of water. A solution at 20 °C that is 0.401 M in MnSO4 monohydrate is best described as a(n) ________ solution. The formula weight of MnSO4 monohydrate is 168.97 g/mol.
Definitions:
Aversive Conditioning
A form of behavioral training in which an unpleasant or painful stimulus is associated with undesirable behaviors to discourage those behaviors.
Systematic Desensitization
A behavioral therapy technique used to reduce phobic responses through gradual exposure to the feared object or situation paired with relaxation exercises.
Free Association
A psychoanalytic technique in which a patient says whatever comes to mind without censorship as a way of uncovering the unconscious mind.
Operant Conditioning
A method of learning that employs rewards and punishments for behavior, reinforcing certain behaviors and dissuading others.
Q13: With what compound will NH<sub>3</sub> experience only
Q18: What types of intermolecular forces exist between
Q21: Crystalline solids differ from amorphous solids in
Q36: The overall order of a reaction is
Q47: When lattice points occur at the corners
Q61: The monomer that is polymerized to make
Q91: On a phase diagram, the critical pressure
Q105: The conjugate base of HSO<sub>4</sub><sup>-</sup> is _.<br>A)OH<sup>-</sup><br>B)H<sub>2</sub>SO<sub>4</sub><br>C)SO<sub>4</sub><sup>2-</sup><br>D)HSO<sub>4</sub><sup>+</sup><br>E)H<sub>3</sub>SO<sub>4</sub><sup>+</sup>
Q120: Sodium bicarbonate is reacted with concentrated hydrochloric
Q132: An aqueous solution contains 0.150 M HCl