The Keq for the equilibrium below is 5.4 × 1013 at 480.0 °C. 2NO (g) + O2 (g)  2NO2 (g)
2NO2 (g)
What is the value of Keq at this temperature for the following reaction?
NO2 (g)  NO (g) +
NO (g) + 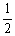 O2 (g)
O2 (g)
Definitions:
Heights
Elevations or areas of high altitude above a point of reference, often causing fear in some individuals.
Adopted Children
Individuals who are legally taken care of by parents other than their biological parents, forming a permanent family bond.
Personality Traits
Enduring characteristics that describe an individual's behavior, such as openness, conscientiousness, extraversion, agreeableness, and neuroticism.
Temperaments
Innate or genetically based aspects of an individual's personality, such as mood, disposition, and reactivity to stimuli, observable from a young age.
Q6: Which statement about steel is false?<br>A)It is
Q9: The concentration of reactants or products at
Q20: An aqueous solution of _ will produce
Q21: Which of the following expressions is the
Q25: The partial pressure of a gas in
Q63: In the _ liquid crystalline phase, the
Q66: Which of the following statements is false?<br>A)The
Q91: Consider the following chemical reaction: H<sub>2</sub> (g)
Q109: The average rate of disappearance of I<sup>-</sup>
Q138: What is the pOH of an aqueous