Given the thermodynamic data in the table below, calculate the equilibrium constant (at 298 K) for the reaction: 2 SO2 (g) + O2 (g)  2 SO3 (g)
2 SO3 (g) 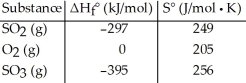
Definitions:
Constant
A fixed value that does not change in mathematical equations or in the context of specific calculations.
Security Market Line
A line that represents the relationship between the expected return of a market security and its risk, as measured by beta.
Market Rate
The market rate, often related to interest or exchange rates, is the prevailing rate determined by supply and demand dynamics in the open market.
Risk-free Rate
The theoretical rate of return on an investment with zero risk of financial loss, often represented by the yield on government securities.
Q3: Which equation correctly represents the reaction between
Q12: Which one of the following pairs cannot
Q13: Classify the following compounds as weak acids
Q19: Write the correctly balanced equation for the
Q28: Consider the following reaction at equilibrium: 2SO<sub>2</sub>
Q76: The K<sub>a</sub> of hydrazoic acid (HN<sub>3</sub>)is 1.9
Q80: Sulfur compounds in the atmosphere are equally
Q89: The standard cell potential (E°)of a voltaic
Q117: Which one of the following substances found
Q179: Which halogen can react with fluorine to