Given the thermodynamic data in the table below, calculate the equilibrium constant (at 298 K) for the reaction: 2 SO2 (g) + O2 (g)  2 SO3 (g)
2 SO3 (g) 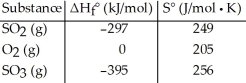
Definitions:
Horizontal Communication
The exchange of information, ideas, or feedback between individuals or groups at the same level in an organization.
Downward Communication
The flow of information from higher levels in an organizational hierarchy to lower levels, often related to directives, goals, or performance feedback.
Social-Emotional Demands
The requirements placed on employees to manage emotions and engage in complex interpersonal interactions as part of their job.
Task Demands
Task demands are the aspects of a job that require physical and mental effort, encompassing the responsibilities, workload, and challenges associated with a particular role.
Q7: _ has the lowest boiling point of
Q61: What is the pH of an aqueous
Q87: The acid-dissociation constant at 25.0 °C for
Q93: Which one of the following reactions is
Q94: Calculate the concentration (in M)of hydronium ions
Q98: Which one of the following is the
Q105: Which group 3A element is a metalloid?<br>A)B<br>B)Al<br>C)Ga<br>D)In<br>E)Tl
Q114: The value of ΔH° for the decomposition
Q122: The oxidation number of N in HNO<sub>3</sub>
Q134: The conjugate base of NH<sub>3</sub> is _.<br>A)NH<sub>2</sub><sup>-</sup><br>B)NH<sub>4</sub><sup>+</sup><br>C)NH<sub>2</sub>OH<br>D)H<sub>3</sub>O<sup>+</sup><br>E)OH<sup>-</sup>