Consider the reaction: FeO (s) + Fe (s) + O2(g) → Fe2O3 (s)
Given the following table of thermodynamic data at 298 K: 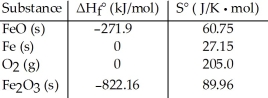 The value K for the reaction at 25 °C is ________.
The value K for the reaction at 25 °C is ________.
Definitions:
Resources Available
The total assets, both tangible and intangible, that are at disposal for use in the production of goods and services.
Production Possibility Frontier
A graph that shows all the maximum production possibilities of two or more products based on certain inputs.
Bowed Out
A phrase indicating withdrawal or retreat from a competition, situation, or commitment.
Increasing Opportunity Costs
The economic concept that as production of a good increases, the opportunity cost of producing an additional unit of that good also increases.
Q2: What is the formula of borax?<br>A)H<sub>3</sub>BO<sub>3</sub><br>B)H<sub>2</sub>B<sub>4</sub>O<sub>7</sub><br>C)P<sub>5</sub>O<sub>8</sub><br>D)B<sub>2</sub>O<sub>3</sub><br>E)Na<sub>2</sub>B<sub>4</sub>O<sub>7</sub> ∙
Q5: The pH of a 0.10 M solution
Q14: Of the following, _ is a weak
Q15: With thermodynamics, one cannot determine _.<br>A)the speed
Q26: Cl atoms formed via photolysis of C-Cl
Q65: Consider a solution containing 0.100 M fluoride
Q72: The relative biological effectiveness (RBE)is tenfold greater
Q79: The effect of a catalyst on a
Q91: A 1.0 × 10<sup>-2</sup> M aqueous solution
Q159: How much energy (in J)is produced when