Given the data in the table below, ΔH°rxn for the reaction 4NH3 (g) + 5 O2 (g) → 4NO (g) + 6 H2O (l)
Is __________ kJ. 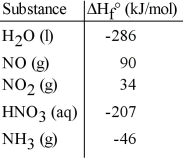
Definitions:
Standard Error
Standard error measures the accuracy with which a sample distribution represents a population, using the standard deviation of the sample means.
Infinite Population
A theoretical concept where the population size is considered to be limitless for the purposes of statistical analysis.
Standard Deviation
A statistical measurement that quantifies the amount of variation or dispersion of a set of values.
Atomic Orbitals
Mathematical functions that describe the wave-like behavior of an electron in an atom, these orbitals determine the distribution of electrons around an atom’s nucleus.
Q17: Which solution has the same number of
Q30: A tenfold dilution of a sample solution
Q45: How many moles of sodium carbonate contain
Q51: What is the empirical formula of a
Q54: This element is more reactive than lithium
Q67: What is the correct formula for ammonium
Q75: The change in the internal energy of
Q85: In the de Broglie formula describing the
Q88: Electromagnetic radiation travels through vacuum at a
Q129: What volume (mL)of a concentrated solution of